from Anten’s Sciences
Zeolite Molecular Sieve The mineral known as “zeolite” or “zeolites” has many different chemical elements in its composition. In general, zeolites are aluminosilicate minerals that can carry water in ….
Zeolite Molecular Sieve

The mineral known as “zeolite” or “zeolites” has many different chemical elements in its composition. In general, zeolites are aluminosilicate minerals that can carry water in their crystalline structure and have the formula M2/nO.Al2O3.xSiO2.yH2O.
Read more: http://www.xmzeolite.com/en/News/industry_news/what-are-zeolite.html
Zeolite molecular sieves are characterized by the following properties:
Chemical Formula: Na2O . AI2O3 . 2Sio2 . 4.5H2O (one of the synthetic zeolites)
Selective adsorption due to the uniform pore size of the zeolite structure
The high adsorption capacity for polar substances at low concentrations
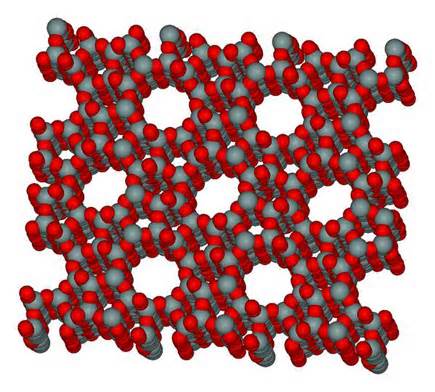
Zeolite – Structure, and Properties
Zeolite molecular sieves are crystalline, highly porous materials, which belong to the class of aluminosilicates. These crystals are characterized by a three-dimensional pore system, with pores of precisely defined diameter. The corresponding crystallographic structure is formed by tetrahedral of (AlO4) and (SiO4). These tetrahedra are the basic building blocks for various zeolite structures, such as zeolites A and X, the most common commercial adsorbents.
Due to the presence of alumina, zeolites exhibit a negatively charged framework, which is counter-balanced by positive cations resulting in a strong electrostatic field on the internal surface. These cations can be exchanged to fine-tune the pore size or the adsorption characteristics.
For instance, the sodium form of zeolite A has a pore opening of approximately 4 Ångstrom (4 x 10–10 m), called a 4A molecular sieve. If the sodium ion is exchanged with the larger potassium ion, the pore opening is reduced to approximately 3 Ångstrom (3A molecular sieve).
On ion exchange with calcium, one calcium ion replaces two sodium ions. Thus, the pore opening increases to approximately 5 Ångstrom (5A molecular sieve). Ion exchange with other cations is sometimes used for particular separation purposes.
The pore opening of the sodium form of zeolite X (13X) is approximately 8 Ångstrom.
The ability to adjust the pores to precisely determined uniform openings allows for molecules smaller than their pore diameter to be adsorbed whilst excluding larger molecules, hence the name “molecular sieve”. The different pore sizes of synthetic zeolites open up a wide range of possibilities in terms of “sieving” molecules of different sizes or shapes from gases and liquids.
Selective Adsorption of Water and other Polar Substances
The uptake of water or other species in zeolites is called adsorption and functions on the basis of physisorption. The main driving force for adsorption is the highly polar surface within the pores. This unique characteristic distinguishes zeolites from other commercially available adsorbents, enabling an extremely high adsorption capacity for water and other polar components even at very low concentrations.
In addition, the pore size plays a significant role, allowing or prohibiting the entrance of molecules to the pore system.
The adsorption on molecular sieves is therefore dependent on the following physical molecular properties:
- Size and Shape: Molecules larger than the pore opening of the molecular sieve can not be adsorbed, smaller molecules can.
- Molecular Polarity: Molecules with large polarity or polarisability can be adsorbed preferentially under identical conditions.
- Of note is the high capacity of Zeolite Molecular Sieves even at low water concentration, allowing them to dry to very low water contents. The Zeolite molecular sieve can retain its high capacity at high temperatures, which makes it the optimal material if drying needs to be carried out at comparatively high temperatures.
The adsorption process is fully reversible and of a purely physical nature. The structure of the zeolite stays intact during the adsorption process (and its later regeneration), and dissolution effects like with other drying agents like calcium compounds can not happen.
Reference
- Zeolite molecular sieves can be used in oxygen production equipment
- New Materials of Toothpastes – Zeolites are used in personal care
- The quality index of the 4A zeolite
- Particle degree distribution of Anten 4A zeolite
- The X ray diffraction of Anten 4A zeolite
- Known more knowledge of other zeolite : natural zeolite of Zeolitemin
