After years of experiments by experts and scholars, it is found that zeolite molecular sieves have better effects when applied to oxygen production equipment. Oxygen concentrator is an industrial structure made by absorbing oxygen from the air through pressure swimming absorption technology or membrane separation method. In the PSA process (Pressure Swing Adsorption), pressurized air is passed through a series of vessels with zeolites as the absorbing membranes. They absorb the nitrogen component of the air and expel oxygen, which is packed in convenient cylinders for medical use.
Zeolite molecular sieves can be applied in oxygen production
Over the past 30 years, experts from Europe, the United States, China, and India have maturely applied this zeolite oxygen production equipment, from understanding the needs and gaps of oxygen cylinders in rural areas, from procurement to installation, guiding the operation process, paving the way for the future, and providing a full range of solutions Health services provide a long-term chain of self-sufficient and sustainable oxygen production methods for the medical and health field.
Oxygen concentrator systems based on zeolite molecular sieves are widely used to produce medical grade oxygen. Zeolites are used as molecular sieves, using their ability to trap impurities to generate pure oxygen from air, leaving behind highly pure oxygen and up to 5% argon in a process that involves the adsorption of nitrogen.
Zeolite molecular sieves can be used in oxygen production equipment
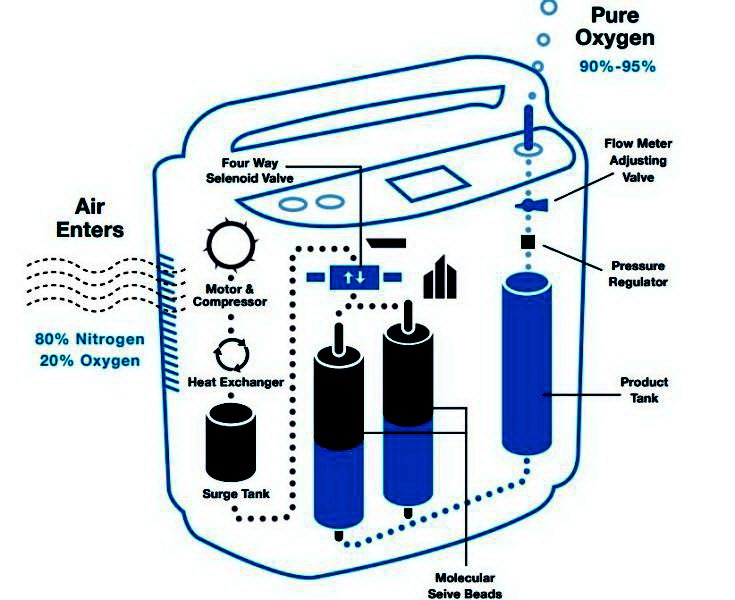
An oxygen concentrator consists of an air compressor, two cylinders filled with zeolite pellets or beads, a pressure equalization vessel, and some valves and piping. Commercial units are capable of delivering up to 10 liters of 90+% oxygen per minute. Oxygen concentrators operate using pressure swing adsorption (PSA) technology, in which air is drawn in, nitrogen is removed and oxygen-enriched gas is released for use by people who require medical oxygen due to low oxygen levels in the blood. So far, no mention has been made of zeolites.
However, they are the heart of the oxygen concentrator. Oxygen concentrators use molecular sieves composed of zeolites to absorb nitrogen in the atmosphere and then discharge the nitrogen. Thus, this type of adsorption system is functionally a nitrogen scrubber, allowing other atmospheric gases to pass through. This leaves oxygen as the dominant gas remaining. Under high pressure, porous zeolites adsorb large amounts of nitrogen due to their large surface area and chemical properties. Afterwards, oxygen and other free components are collected. The pressure drop allows the nitrogen to desorb. Zeolites act as filters for nitrogen molecules in a simple way.
Zeolites are widely used in PSA systems due to their ability to discriminate between different gases and their large specific surface area. Furthermore, their porosity plays a key role in the adsorption process. The regularly arranged pores and cavities (micropores) generated by the assembly of SiO4 and AlO4 tetrahedral structures enable some molecules to be selectively absorbed in the micropores while others are rejected due to steric effects or differences in affinity, such as oxygen passing through Oxygen concentrator with nitrogen adsorbed on zeolite.
